If you have patients with SCN2A or SCN8A related DEE, they may be eligible for the EMBOLD Study. You can find more information below and at www.emboldstudy.com.
Eligible families can self-enrol through the study website.
Roger Rolph from Praxis Medicines had hoped to present the study at our February 2025 meeting but technical issues on the day prevented him from doing so. Instead, he has shared this information and has indicated that he is happy to organise 1:1 Microsoft Teams meetings with any clinician who may have eligible patients by prior arrangement over e-mail.
- 2 UK sites planned – London and Scotland, study MHRA approved
- All study visits can take place in the patients’ homes
- Decentralised study design
- SCN2A and 8A, ages 2-18 years.
- 80 places available globally
- Therapeutic dose from day 1, no titration
- Daily liquid dose 1mg/kg orally or via G/J-tube
- 2 sodium channel agents allowed in addition to relutrigine
- 4-week seizure diary prior to active drug phase (8 motor seizures per 4 weeks min)
- 2 groups – 16-weeks active drug or 12-weeks active drug + 4 weeks placebo
- 52-week Open Label Extension available plus enhanced patient access for those with meaningful improvement
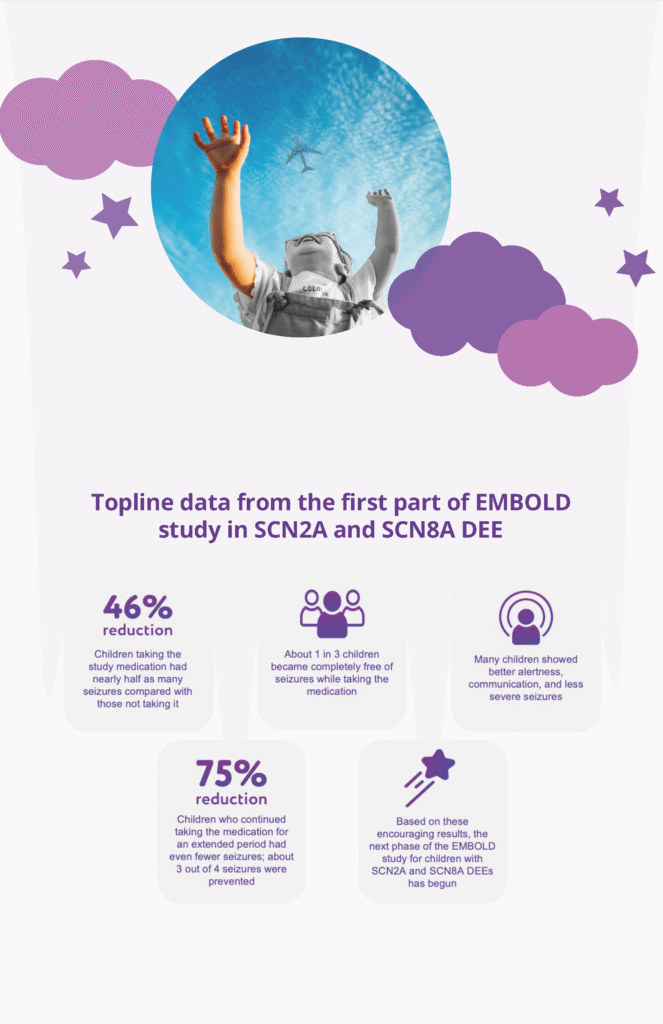
- Phase 2 results from 15 pts on the attached flyer – we are seeking to replicate in Phase 3 study of 80 pts